Following the octet rule results in completely filled s- and p- orbitals in an atoms outermost energy level. There are many compounds that do not follow octet rulethe rule that suggest that every element gets stability by acquiring eight electrons in outermost or valance shell but here I will quote the example of PCl5phosphorous penta chlorideIn PCl5 P atom has 5 electrons in outermost or valance shellso in order to complete its octetit must form 3 bonds as in case of PCl3 but it.
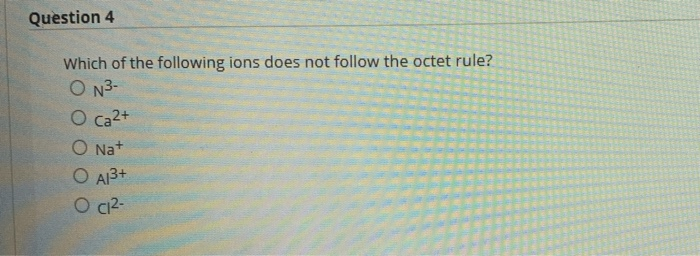
Solved Question 4 Which Of The Following Ions Does Not Chegg Com
The octet rule shows that a full principle energy shell is naturally lower.

. An electron or molecule which contains unpaired electrons in its outermost shell or valence shell is considered a free radical. These molecules called free radicals contain at least one unpaired electron a clear violation of the octet rule. However there are atoms which only have the first shell which can only hold 2 electrons on it like ions Li Be2 and H-.
Some elements that disobey the octet rule include. The majority of molecules or complex ions discussed in general chemistry courses are demonstrated to have pairs of electrons. On the other hand some elements exhibit hypervalency and have the ability to form hypervalent molecules.
These electrons are less stable and do not obey the octet rule. The ions Li Be2 and H- do not follow the octet rule. Things like Sodium Na and Hydrogen.
In the case of normal polyatomic ions an electron is gained to facilitate the general reduction in energy for other electrons in the molecule. Simply because once bound in a state of lower potential it takes energy to return the electron to a state of higher potential. Boron has three valence electrons.
An exception to an octet of electrons is in the case of the first noble gas helium which only has two valence electrons. Question 4 Which of the following ions does not follow the octet rule. Atoms follow the octet rule because they always seek the most stable electron configuration.
Yes there are many molecules such as sulfur hexafluoride which do not follow the octet rule. Some of the exceptions to the octet rule are given below. Many elements do not follow the octet rule.
Hydrogen beryllium and boron have too few electrons to form an octet. Which ions do not follow the octet rule. Low atomic weight elements the first 20 elements are most likely to adhere to the octet rule.
Are there any molecules that do not follow the octet rule. Transition metal cations usually have electrons in d orbitals. Chemistry The Practical Science- Study Guide to Accompany 1st Edition Edit edition Solutions for Chapter 8 Problem 3E.
Why Elements Follow the Octet Rule. Having an odd number of electrons in a molecule guarantees that it does not follow the octet rule because the rule requires eight electrons or two for hydrogen around each atom. The most commonly encountered stable species that exist with an odd number of electrons are nitrogen oxides such as nitric oxide NO and nitrogen dioxide NO 2 both of which are free radicals.
Elements like hydrogen lithium helium do not obey. This primarily affects the element hydrogen which forms stable. However there are a few stable molecules which contain an odd number of electrons.
Elements that do not have 8 electrons to make an octet. ICl_2-1 There are 22 electrons shared between 3 atoms. The octet rule states that atoms tend to form compounds in ways that give them eight valence electrons and thus the electron configuration of a noble gas.
Free radicals play many important roles a. List Four Elements that do not Obey the Octet Rule. Since 1p subshells do not exist some elements find stability in 1s 2 configurations.
Transition metal cations often do not follow the octet rule. ON3- O ca2 O Na O A13 O C12- ON3- O ca2 O Na O A13 O C12- This problem has been solved. NO does not follow the octet rule Nitrogen starts with five electrons gains two from Oxygen but ends with only 7.
They are stable with 2 electrons only Some definition of the octet rules states that atoms would like to have 8 electrons in its valence shell. The Boron ends up with no electrons in the outer shell The complete inner shell is the same as group VIIIA helium. Beryllium has only two valence atoms and can form only electron pair bonds in two locations.
Hydrogen has only one valence electron and only one place to form a bond with another atom. Give three examples of elements or ions that do not follow the octet rule but instead follow the duet rule.

Exceptions To The Octet Rule Boundless Chemistry

Solved Question 4 Which Of The Following Ions Does Not Follow The Octet Rule N3 Ca2 Nat A13 Ci2
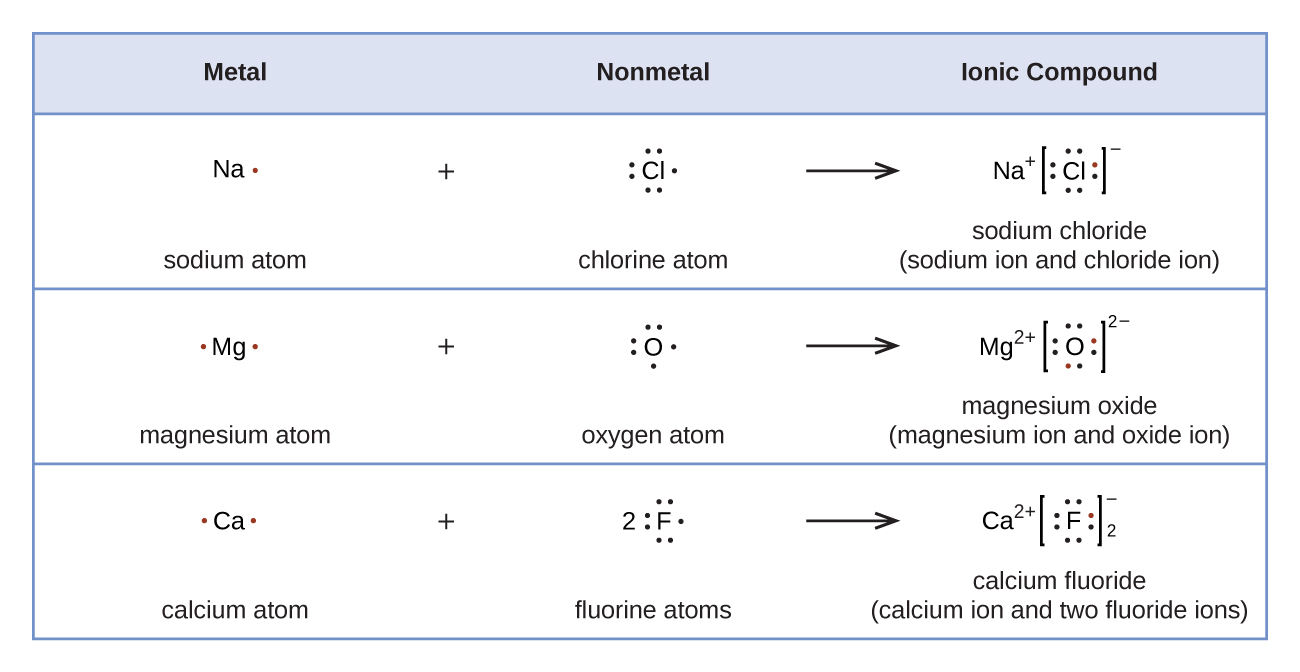
10 1 Lewis Structures And The Octet Rule Chemistry Libretexts

Solved Which Of The Following Molecules Ions Does Not Obey Chegg Com
0 Comments